Indications (ACC/AHA Guidelines)
Hemodynamically significant renal artery stenosis (RAS) with recurrent unexplained heart failure, sudden explained pulmonary edema, or unstable angina
Hemodynamically significant RAS = >50% stenosis and pressure gradient >10% of systemic SBP, urine albumin to creatinine ratio < 22.5 mg/g, peak systolic velocity >220 cm/s or aortic to renal artery peak systolic velocity of >3.5
RI <0.8 is predictive of greater benefit
RAS with resistant hypertension and intolerance to medication or progression of CKD
RAS to solitary functioning kidney
Etiologies of native renal artery stenosis: atherosclerosis (often involves the proximal third of the artery) > fibromuscular dysplasia (FMD, often involves the mid to distal third) > neurofibromatosis type 1, polyarteritis nodosa, takayasu arteritis, radiation arteritis.
Etiologies for transplant renal artery stenosis: para-anastomotic > pre- or -post-anastomotic; related to suture technique, clamp injuries, or kinking
Contraindications
Uncorrected coagulopathy (INR >1.5, Plt <50K)
Unfavorable anatomy
Efficacy and alternatives
Technical success of renal artery stenting and/or angioplasty is ~90%
Clinical success:
Reduced hypertension: 90-100% for FMD, 85% for Takayasu, 50-90% for atherosclerosis (33-50% cured, 33% improve, 15-20% no change), 70-100% for transplant anastomotic stricture
Reduced azotemia: 81%
In-stent restenosis: 15% in 1 yr, 20% in 3 yrs (native renal artery) vs >90% at 2 yrs (transplant renal artery)
For FMD, angioplasty alone is superior to stenting
Endovascular management vs. Surgery:
Fewer major complications (3-11 v. 20%) and 30d mortality (<1 v. 5.9%).
With surgical intervention, 18% are cured, 71% improve, 11% are unchanged or worse
Endovascular management vs Medical therapy:
Majority of older studies have shown no statistically significant benefit over medical therapy for hypertension, creatinine, major CV/reenal events, or mortality (ASTRAL and CORAL trials). There was possible greater decreased DBP and less anti-hypertensive use, but overall no benefit (meta-analysis and Cochrane review)
However, newer studies have shown benefit of radiofrequency renal denervation in RADIANCE II and SPYRAL HTN-ON MED with an average of 8-20 mmHg SBP decrease.
Pre-procedure care
Review available imaging - often diagnosed on US, CTA, or MRA. Cross sectional imaging is helpful for procedure planning but not strictly necessary.
Discontinue antihypertensives if possible and give 10 mg nifedipine PO 1-2 hrs prior to prevent vasospasm
Hydration for GFR 30-60
procedure
Obtain access, often ipsilateral or contralateral CFA, 5-7 Fr pending intended devices to be used.
Initial aortogram or iliac angiogram for transplant renal artery stenosis. Can use CO2 and save contrast for renal artery run for intervention planning.
Advance guide catheter or long curved sheath (see below) over a working wire to renal artery ostium and perform an initial run and characterize the anatomy. This may require runs in 2-3 projections to best lay out the vasculature.
It is often good to use iodinated contrast sparingly given these patients’ compromised renal function. CO2 can be used instead, though most will still use iodinated contrast for at least one run for stent sizing.
Some administer heparin prior to gaining renal artery access. Others wait until just prior to treatment treatment (e.g. 70-100 U/kg)
Gain wire access across the targeted lesion
Often a curved catheter (e.g. RDC, C2, Sos, or Simmons) and soft guidewire +/- a long curved sheath if additional stability is needed for stent positioning.
If plan for angioplasty alone, a short access sheath and curved catheter is often sufficient.
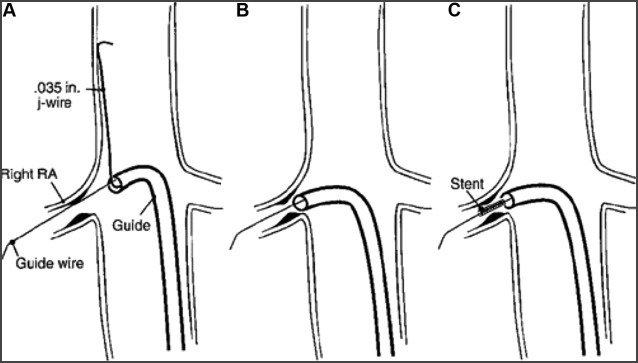
If treating an ostial atherosclerotic stenosis, many recommend the “no touch technique” to avoid disrupting the plaque and causing distal emboli (see image below). A curved sheath or guide catheter is advanced over a J-tip 0.035” wire passed the renal artery ostium in the suprarenal abdominal aorta to perch the sheath/catheter pointed towards the ostium. A guidewire is used to select the target renal artery through the sheath/catheter. The J-tip 0.035” wire is removed allowing the sheath/catheter to move gently engage and be seated in the ostium.
(Optional) Pre-intervention pressure gradient measurement across the stenosis.
Angioplasty +/- stenting of the target lesion
Advance catheter beyond the target lesion and exchange for stiff working wire such as an Amplatz with 1 cm flop guidewire or steel core or spartacore if using 0.018” or 0.014” wires. Many prefer these wires to J-tip wires that can cause spasm. Must meticulously watch the wire tip, which can migrate distally and perforate the renal capsule.
Angioplasty often sized 1:1 or 10% oversize. If severe stenosis, may require serial plasty. Often done for FMD as well as transplant anastomosis.
Stenting often done for atherosclerotic stenosis and refractory stenoses. Traditionally has been done with balloon expandable stents for greater precision with deployment (e.g. covered iCAST stent) including off-label drug-eluting coronary stents for smaller arteries. May require predilation with undersized balloon to advance stent and prevent watermelon seeding of the stent during deployment. For ostial lesions, the stent should ideally just protrude into the parent vessel lumen. Some over dilate this endto flare the stent.
Long-term outcomes in terms of stent patency are superior with final diameters of 6 mm or greater.
Repeat angiography to assess need for further intervention.
(Optional) Post-intervention pressure gradient measurement across the stenosis.
Achieve hemostasis.
No Touch Technique
Radiofrequency Renal Denervation:
Similar access to above but requires anesthesia.
Advance RF probe (e.g. Simplicity, Medtronic) through curved sheath into the renal artery.
Normal renal artery of minimum of 2 cm, ideally single renal artery at least 4 mm in diameter
RF treatment in 4-6 locations between the renal hilum and ostium in spiral pattern.
Complications
Anngioplasty alone 5-10% vs stenting 15% overall
Vessel dissection (4%)
Peripheral renal emboli (2%)
Exacerbation of renal failure (1.5-6.0%)
Emboli to extremities (1.5-2.0%)
Myocardial infarction (1%)
Renal artery thrombosis or rupture (<1%)
Post-procedure care & Follow Up
Close BP monitoring for 24-48 hours.
People vary but some will use DAPT with aspirin and clopidogrel for a period of time after stenting and then transition to daily aspirin alone.
Renal artery doppler US in 6 weeks, 6 months, 12 months, and then annually